|
Oceans
The oceans contain mountains taller than Everest, canyons deeper
than the Grand Canyon, and the largest animals on earth. The oceans
are home to 97% of all of the life on earth. The most important
difference between the earth and the other planets in our solar
system is the existence of water in liquid form. It is the source
of all life as we know it. In fact, our bodies are made up mostly
of water and the tears from our eyes are the same consistency
as seawater. When you consider the fact that life in the oceans
can be found from the surface all the way down to the very bottom
of the deepest ocean trench, it is not surprising to realize that
the oceans represent over 99% of the living space on Earth....we
are indeed living on what is truly an Ocean Planet.
One recent study of a deep-sea community revealed 898 species
in an area about half the size of a tennis court. More than half
of these species were new to science! And, consider the fact that
there exists a creature in the oceans that we know to be the world's
largest invertebrate, that can grow to at least 60 feet in length,
has eyes as large as volleyballs, but which has never been seen
alive in its natural habitat.
The oceans, driven by the sun's heat, control our weather and
maintain the temperature of our world. The oceans are as important
to our survival as the air that we breathe. On the other hand,
as powerful as the oceans are, they are equally as fragile. Threats
to the health of our oceans equate to threats to our entire planet,
and to us.
What color is the ocean? 
Why are phytoplankton so important? 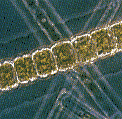
What is carbon dioxide and what does it have
to do with the oceans? 
What are the primary threats to the oceans as we know them today?
When you're finished reading, try the exercises.
To learn about the creatures who live in the ocean, check out
the Sealife page.
To learn about rivers, check out the Rivers
page.
What Color
is the Ocean?
Did you know that the ocean isn't just blue? 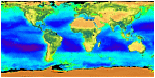
If someone were to ask you what the color of the ocean was, chances
are that you would answer that is was blue...and for most of the
world's oceans, your answer would be correct. But...it's a little
more complicated than that.
We see color when light is reflected by the things around us.
White light is made up of a spectrum, or combination of colors,
as in a rainbow, of many different wavelengths. The longer
wavelengths of light are red and the shorter wavelengths are blue.
The order of the colors in the rainbow - Red, Orange, Yellow,
Green, Blue and Violet (ROYGBV) - reflect the order of their wavelengths,
from longest to shortest.
When light hits the surface of an object, these different colors
can be reflected or absorbed, in differing amounts, depending
on the unique properties of the material on which the light is
shining. So, the color we see depends on which colors are reflected
and which colors are absorbed. For example, a book that looks
red to us absorbs more of the green and blue parts of the white
light shining on it, and it reflects the red parts of the white
light. The same kind of thing thing happens when we look at the
ocean.
When sunlight hits the ocean, some of it is reflected back directly
(called sunglint), but most of it penetrates the ocean surface
and interacts with the water molecules that it hits. Most of the
light that is scattered back out of clear, open ocean water is
blue, while the red portion of the sunlight is quickly absorbed
very near the surface. So usually, the ocean looks blue to us.
However, there are many things in addition to just water molecules
in the ocean. It is these other things that can change the color
that we see.
In coastal areas, runoff from rivers, sand and silt stirred up
from the bottom by tides, waves and storms, and a number of other
things can change the color of the near-shore waters and make
it look brown or tan or even dark green.
However, for most of the world's oceans, the most important things
that influence the color of the ocean that we see are phytoplankton.
Phytoplankton are very small (smaller than the size of a pinhead!)
single-celled ocean plants that contain a green pigment called
chlorophyll. All plants (on land and in the ocean) use
chlorophyll to capture energy from the sun. Through the process
known as photosynthesis, these teeny-tiny ocean plants convert
water and carbon dioxide into new plant material and, importantly
for us, into the oxygen that we need to breathe to stay alive.
Although each phytoplankton by itself is tiny, they can reproduce
and "bloom" in such large numbers that they can change
the color of huge areas of the ocean. We can even measure that
ocean color change all the way from space.
Satellites use special instruments that are even more sensitive
than the human eye. With them, we can measure the fantastic array
of colors of the ocean. Because different types of phytoplankton
have different amounts of chlorophyll, they appear as different
colors to these sensitive satellite instruments. When we look
at pictures of the ocean that are taken by these satellites, we
see many different shades of blue, of course, but we also see
other colors such as green, yellow and red. These different colors
show us where there is phytoplankton, sediments, and other chemicals.
This helps us to understand what is in the oceans, and how healthy
the ocean is. Comparing pictures taken at different times shows
us what is changing in the oceans' makeup and health.
One satellite that is used to take pictures of the ocean from
space is called the Sea-viewing
Wide Field-of-View Sensor (SeaWiFS). Their Website has many
interesting and beautiful pictures that SeaWiFS has taken of the
ocean.
Why are
Phytoplankton so Important?
These tiny little plants that drift with the currents throughout
the ocean play an absolutely critical role for all life on this
planet. You may ask "why should I care about a bunch of tiny little
floating plants in the ocean that I can't even see, and what could
they possibly do for me?"
Well, it is safe to say that without phytoplankton, life on earth
as we know it would never have happened and without them, life
on earth as we know it would cease to exist. That's pretty important!
Just to give you a few facts to whet your appetite:
- A teaspoon of sea water can contain as many as a million one-celled
phytoplankton
- The world's phytoplankton generate at least half of the oxygen
we breathe
- Over 99.9% of all the carbon dioxide that has been incorporated
into living things over geologic time is buried in marine sediments,
and most of that was done by phytoplankton
- Phytoplankton are the base of the marine food web and therefore,
with a few fairly unusual exceptions, they support almost all
life in the oceans
These small plants are the beginning of the food chain for most
of the planet. As phytoplankton grow and multiply, small fish
and other animals eat them as food. Larger animals then eat these
smaller ones. The ocean fishing industry finds good fishing spots
by looking at ocean color images to locate areas rich in phytoplankton.
The ocean color pictures taken by satellites
show scientists where ocean currents are full of phytoplankton.
Depending on their color, they can also show where pollutants
poison the ocean and prevent plant growth. Even subtle changes
in the climate, warmer or colder, more saline (salty) or less
saline, affect phytoplankton growth. Since phytoplankton depend
upon very specific conditions for growth, they often are the first
indicators of a change in their environment.
What is
Carbon Dioxide?
And What does it Have to do With the Oceans, Anyway?
Carbon dioxide is one of the gases in our atmosphere that helps
to maintain the earth's temperatures. Although it is colorless
and odorless, we may notice it because it is the gas used to make
the fizz in our soft drinks. It is also used as a refrigerant
(dry ice is solid CO2), and in fire extinguishers. Plants, including
phytoplankton in the oceans, use carbon dioxide to make the oxygen
that we breathe. When we (and all animals) exhale, carbon dioxide
is one of the gasses that comes out of our lungs. Scientists use
the notation CO2 (pronounced see-oh-two) to represent carbon dioxide.
Click here for a diagram
of the Carbon Cycle.
Phytoplankton are a critical part of ocean chemistry and of the
world's food and sources. As we learned above,
phytoplankton acts as the first link in the ocean's food chain.
In addition, during photosynthesis, phytoplankton remove carbon
dioxide from sea water and release oxygen as a by-product. This
allows the oceans to absorb additional carbon dioxide from the
atmosphere. If fewer phytoplankton existed, atmospheric carbon
dioxide would increase. If fewer phytoplankton existed, the web
of life in the sea would shrink.
Phytoplankton also affect carbon dioxide levels when they die.
Phytoplankton, like plants on land, are composed of substances
that contain carbon. Dead phytoplankton can sink to the ocean
floor. The carbon in the phytoplankton is soon covered by other
material sinking to the ocean bottom. In this way, the oceans
act as a sink, a place to dispose of global carbon, which otherwise
would accumulate in the atmosphere as carbon dioxide.
Other global sinks include land vegetation and soil. However
the carbon in these sinks frequently is returned to the atmosphere
as carbon dioxide by burning or decomposition. Deforestation contributes
to the accumulation of carbon dioxide in the atmosphere by reducing
vegetation that takes up carbon dioxide.
Carbon dioxide acts as a "greenhouse gas" in the atmosphere,
and therefore may contribute to global warming.
Sources of carbon dioxide in the Earth's atmosphere include decomposition
of organic matter (such as trees), the carbon dioxide that animals
and people exhale, volcanic activity, and human activities such
as the burning of fossil fuels and wood. No one yet knows how
much carbon the oceans and land can absorb. We also do not know
how the Earth's environment will adjust to increasing amounts
of carbon dioxide in the atmosphere. Studying the distribution
and changes in global phytoplankton using ocean color and other
tools will help scientists find answers to these questions. You
can learn more about the ocean colors project at the Sea-viewing
Wide Field-of-View Sensor (SeaWiFS) Website.
Ocean
Pollution and its Sources
While pollution comes from visible sources like oil spills and
trash, many little known sources have big impacts as well. These
include polluted runoff, invasive species, marine deforestation,
careless boaters and ship operators, and many others. Even fertilizers
and pesticides used on lawns and farms contribute greatly to the
pollution of the oceans.
During recent years the number of beach closing and advisory
days have topped 11,000 per year in the United States alone. Many
more beaches are polluted around the world, but they are not tested
or reported regularly. There are several culprits behind this
rampant pollution.
As rain water washes over roads, parking lots, construction sites
and commercial and industrial sites, it becomes contaminated with
oil and grease, heavy metals, pesticides, litter and pollutants
from vehicle exhaust. This polluted runoff flows into storm drains
and eventually most of it ends up in streams, rivers and coastal
waters. In fact, about 25% of our nation's polluted estuaries
and lakes are fouled by urban storm water. In rural areas, rainwater
flows over farmland, roads, golf courses and lawns into waterways.
The rainwater can become a toxic mix of animal waste, fertilizers
and pesticides.
When too many homes and businesses are hooked up to a sewage
treatment plant, it can't treat the sewage adequately, especially
after heavy rainstorms. The overburdened facilities can, and often
do, malfunction. Under these circumstances, untreated wastewater
(from emptying sinks and showers, and all the stuff flushed down
toilets) is released into local waterways.
Floating debris regularly washes up on the shores of places as
remote as Antarctica, and plastic bottles and bags are routinely
seen floating in mid-ocean. In a single day in 2000, 850,000 people
removed 13.6 million pounds of debris from the world's beaches
and coastal waters. On that same day, volunteers discovered nearly
13,000 syringes on the worlds beaches, and found 373 dead marine
animals entangled in debris.
Cruise Ships
Floating cities with their own zip codes, cruise ships carry millions
of people each year to some of the world's most pristine and sensitive
ecosystems. Largely unregulated, cruise ships also discharge huge
amounts of waste directly into the water. From raw sewage to toxic
chemicals, cruise ships dump huge amounts of waste directly into
ocean waters, posing a potential threat to marine wildlife, fragile
habitats and human health. Cruise ship impacts have increased
exponentially with the industry's dramatic growth. In 1998, 223
cruise ships carried 10 million passengers through some of the
world's most sensitive ocean ecosystems. Since then, the industry
has grown by an average of 10 percent annually, and is expected
to bring more than 49 new vessels into service by 2005.
Solutions
If you want to help bring an end to the pollution problem, there
are three things we can all do.
- Most importantly, try not to be a part of the problem.
--Remember that virtually every chemical we use ultimately ends
up in the ground water. So, ask your parents to only use soaps
and cleansers that are biodegradable and don't contain bleach
or antibacterial cleaners.
--Avoid the use of pesticides and fertilizers on your lawn at
home and make sure all of the plastic packaging from the products
you do buy are properly disposed of.
--Never let a helium balloon fry free into the atmosphere, because
they bust and fall back to earth, where many animals confuse
them with food, eat them, and choke to death.
- Volunteer to help out in cleaning up your community on Earth
Day.
Every piece of trash you pick up potentially saves the life
of an animal somewhere down the line and makes your world just
a little cleaner.
- Write the government officials on every level who represent
you and your community and ask them what they are doing to help
with this problem.
Overfishing
and Whaling
Once vibrant wildernesses teeming with diverse marine life, many
of today's ocean ecosystems face the devastating effects of overfishing.
A number of species have gone commercially or ecologically extinct,
causing dramatic changes in ocean ecosystems. Rebuilding over-fished
species will actually increase the amount of fish that can be
caught sustainably.
Overfishing occurs when fish are caught faster than they can
reproduce, so their overall population declines. Many marine scientists
now believe that overfishing is the biggest human impact on the
world's oceans. A recent study in the prestigious journal Science
showed that overfishing makes ocean ecosystems more vulnerable
to harm from other human impacts like pollution.
Evidence of overfishing abounds throughout US waters, including
the near-disappearance of fish that were once abundant, and the
shrinking sizes of average-sized fish. Today, many fish are caught
before they are old enough to reproduce. Overfishing also can
contribute to declines in marine bird and mammal populations by
reducing their food supplies.
Depletion of fish populations is actually an accepted goal for
most fishery managers. Fishermen are encouraged to achieve maximum
exploitation by "fishing down" populations to about half their
original size. Such goals ignore the role of fish as an integral
part of marine food webs.
Whaling
Valued for their oil, blubber, meat and baleen, many species of
whales were hunted nearly to extinction by the 20th century. Even
with laws enacted by the International Whaling Commission (IWC),
most species haven't recovered. Some countries, like Japan and
Norway, still ignore conservation plans, threatening the survival
of these awesome animals.
Several scientific concerns must be addressed before the resumption
of commercial whaling is even considered, including:
- increasing our knowledge of stock structure to ensure minimal
damage and sustain genetic diversity for each species
- gathering reproductive data to determine overall sustainability
of each species
- studying the cumulative impacts of environmental variables
on each species, including contaminants and prey availability
In addition, as past experience has revealed, ensuring responsible
whaling by all nations is a constant struggle due to inadequate
enforcement and monitoring capabilities.
Entanglement
From dolphins to whales, sea turtles to sea lions, thousands of
animals die each year entangled in commercial fishing gear or
in abandoned fishing nets and lines, and in marine debris. Fishing
line and nets, rope and other rubbish can wrap around fins, flippers
and limbs-causing drowning, infection, or amputation. Several
species of dolphins, porpoises and whales are particularly vulnerable
to entanglement. Often, these species feed on the same fish that
nearby humans are fishing. Other times, they swim with, feed near,
or inhabit the same area as those fish.
The Marine Mammal Protection Act (MMPA) provides the only defense
for these species. It requires that teams of specialists develop
mechanisms to reduce entanglements and to ensure the continued
health of marine mammal populations.
Global
Warming
Energy from the sun drives the earth's weather and climate, and
heats the earth's surface; in turn, the earth radiates energy
back into space. Atmospheric greenhouse gases (water vapor, carbon
dioxide, and other gases) trap some of the outgoing energy, retaining
heat somewhat like the glass panels of a greenhouse. Without this
natural "greenhouse effect," temperatures would be much lower
than they are now, and life as known today would not be possible.
Without the Earth's atmosphere, our planet would become extremely
cold and barren of life. Instead, thanks to greenhouse gases,
the earth's average temperature is a more hospitable 60°F. However,
problems may arise when the atmospheric concentration of greenhouse
gases increases.
The atmosphere consists of nitrogen (about 70 percent) and oxygen
(about 20 percent). The other ten percent consists mostly of carbon
dioxide, water vapor, and several "trace" gases such as neon and
argon.
Like the glass roof and walls of a greenhouse, the Earth's atmosphere
keeps its surface much warmer than it would be without the "greenhouse
effect." How?
Energy from the sun arrives as short-wavelength radiation (light),
while the Earth emits long-wavelength (infrared) energy back into
space. The hotter an object is, the shorter the wavelength of
the radiation it emits. The short-wavelength sunlight easily penetrates
the atmosphere and warms the Earth. However some of the long-wavelength
energy emitted from the Earth is absorbed by the atmosphere before
it escapes into space.
Carbon dioxide, water vapor and other gases in the atmosphere
are responsible for absorbing escaping long-wavelength energy.
Thus, the Earth keeps some of the heat that would otherwise have
been lost to space.
The concentration of carbon dioxide in the atmosphere has changed
in the last hundred years. Before the Industrial Revolution, carbon
dioxide levels stayed nearly stable for thousands of years. Since
human beings developed a fossil-fuel- based global economy and
lifestyle, the amount of atmospheric carbon dioxide has increased
dramatically. Since the beginning of the industrial revolution
to 2004, atmospheric concentrations of carbon dioxide have increased
nearly 35%, methane concentrations have more than doubled, and
nitrous oxide concentrations have risen by about 15%. This increase
means that less long-wavelength energy emitted from the Earth
can escape to space. These increases have enhanced the heat-trapping
capability of the earth's atmosphere. The heat-trapping property
of these gases is undisputed, although uncertainties exist about
exactly how earth's climate responds to them.
Why are greenhouse gas concentrations increasing? Scientists
generally believe that the combustion of fossil fuels and other
human activities are the primary reason for the increased concentration
of carbon dioxide. Plant respiration and the decomposition of
organic matter release more than 10 times the CO2 released by
human activities; but these releases have generally been in balance
during the centuries leading up to the industrial revolution,
with carbon dioxide absorbed by terrestrial vegetation and the
oceans.
Many scientists believe this increase in the heat-trapping capability
of the earth's atmosphere can lead to a gradual warming of the
Earth, but others believe that different factors counteract this
warming effect. For example, cloud cover reflects sunlight before
it ever reaches the Earth, thus reducing the amount of sunlight
that reaches the Earth's surface. For another example, sulfate
aerosols, a common air pollutant, cool the atmosphere by reflecting
light back into space, but sulfates are short-lived in the atmosphere
and vary regionally. Studying these processes is difficult, because
they are complicated.
Ocean color information provides one of the many tools scientists
use to try to find what changes are occurring, and how they may
affect us. According to the National Academy of Sciences, the
Earth's surface temperature has risen by about 1 degree Fahrenheit
in the past century, with accelerated warming during the past
two decades. There is new and stronger evidence that most of the
warming over the last 50 years is attributable to human activities.
Rising global temperatures are expected to raise sea levels and
change precipitation and other local climate conditions. Changing
regional climates could alter forests, crop yields, and water
supplies. It could also affect human health, animals, and many
types of ecosystems. Deserts may expand into existing range lands,
and features of some of our National Parks may be permanently
altered.
Most of the United States is expected to warm, although sulfates
may limit warming in some areas. Scientists currently are unable
to determine which parts of the United States will become wetter
or drier, but there is likely to be an overall trend toward increased
precipitation and evaporation, more intense rainstorms, and drier
soils.
Unfortunately, many of the potentially most important impacts
depend upon whether rainfall increases or decreases, which can
not be reliably projected for specific areas. However, most scientists
agree that this Greenhouse effect is responsible for accelerating
the melting of the polar ice cap, which is changing the temperature
of our oceans. This has devastated coral reef formations around
the world, which is home to innumerable species of marine life.
A change of only 1-2 degrees can destroy these delicate formations.
Exercises
1, There are areas of the ocean with relatively large concentrations
of nutrients that animals and plants use as food substances. In
these areas you see a lot of phytoplankton the plant portion of
plankton), especially in the spring. Why do some areas have greater
amounts of phytoplankton? Where would be the best place for deep-sea
fishing?
2. If a zooplankton, a very small animal type of plankton, eats
a phytoplankton, generally speaking, what happens to the zooplankton
and the carbon that remained in the phytoplankton?
3. What is an example of the lowest level of the "food chain"
on land?
4. Scientists use two types of satellites to study the environment.
A geostationary satellite remains above the same spot on the Earth's
equator from an altitude of about 22,500 miles, and can "see"
an entire hemisphere all the time. A polar orbiting satellite
travels in a circular orbit, passing above the North and South
Poles while the Earth rotates beneath it. This type of satellite
can "see" details as small as a mile or less. Which of these satellites
probably would be better for our ocean color instrument? Would
one prove better than the other to track hurricanes and other
large weather systems?
5. How do the atmosphere and the ocean interact?
6. How could global warming affect sea levels? Why is global
warming important?
7. Where do plankton grow?
Project: Make a Greenhouse
Materials needed:
- Two cardboard shoe boxes
- Clear plastic wrap
- Two regular "weather type" thermometers
- Desk light with 15 watt or larger bulb
Procedure:
1. Place some paper towels loosely in the bottom of each shoe
box, then lay the thermometer on the towels.
2. Cover the open top of one box with plastic wrap, taped to
the side of the box; leave the other box with the top off (open).
3. Place the boxes side by side, and move the desk light so it
shines evenly into both boxes.
4. Record the temperature in each box every minute for 10 minutes.
5. Plot the temperatures on a graph with time as the "x" (horizontal)
axis and temperature as the "y,' (vertical) axis.
Analysis
Discuss the differences you see in the observed temperatures in
the two boxes, and why this is happening.
Variation
Try replacing the paper towels in each box with black paper. Repeat
the experiment. What differences do you note?
|
![]()